Shop Products
Custom Preclinical Research Services
Custom Preclinical Research Services
Contract research organizations, or CROs, fulfill outsourcing contract research needs for large biotechnology and pharmaceutical companies. Biology CROs provide various preclinical research and development services from study design through IND regulatory submissions. Preclinical studies require specific research area knowledge and experimental expertise, as well as laboratory quality control systems where preclinical experiments are conducted. We provide cost efficient and high-quality laboratory research services for all stages of drug development and preclinical testing. Our services are often utilized for generation of IP (our company policy is that 100% of intellectual property belongs to the client) for regulatory (IND) and patent applications.
Quality Control Systems
Usually companies outsource specific lab work to trusted laboratories because of the need for reliable data. This reliability is ensured through quality control systems that are outlined in FDA regulations pertaining to clinical trials. For laboratories, the general standard is the Good Laboratory Practice (GLP) system, which is outlined by the FDA. GLP standards are incredibly strict, and require large amounts of experimental quality control, data gathering, analysis, and precision. GLP-compliance is necessary for drug testing in pre-clinical trials, which is why companies often outsource their testing requirements to GLP-compliant laboratories. The widespread effective solution to reliable drug testing has been to use contract research laboratories with GLP certification (such as Altogen Labs CRO).
GLP certification is an advantage that guarantees reliable results. However, GLP-certified research is expensive, and it can often be more useful to have basic outsourced research that brings quick results that are used for further development. As such, contract research organizations can adapt to client preferences and conduct reliable studies that do not necessarily require GLP-compliance. The results obtained from such studies are still reliable, but can be achieved quicker and cheaper if a company needs results for initial decisions (such as deciding whether or not continue testing a drug in vitro).
CRO Activities
Biology CROs work closely with their clients, advising them on preclinical experimental design, compound testing strategies, protocols, data management and report preparation. Whether it is drug discovery, drug development, or protein manufacturing, partnering with a CRO can result in significant cost-savings and a more streamlined process towards bringing a product to market. Particularly in the early stages in the drug or medical device development process, a large amount of analytical data must be acquired to validate the usefulness of the product. Preclinical studies are important because they impact the overall development pathway for an investigational product. Preclinical studies establish biological plausibility, dosage levels, safety, support patient eligibility criteria, and may identify potential public health risks.
What is Preclinical (Nonclinical) Research Services
Preclinical research refers to laboratory studies and animal testing conducted before human clinical trials to evaluate the safety, efficacy, and toxicity of new drugs, treatments, or medical devices. Preclinical research is an important step in the drug development process, as it provides crucial data on the potential benefits and risks of a new treatment before it is tested in human subjects.
Preclinical studies typically involve the following steps:
- Identification of a target: The first step in preclinical research is to identify a target for a new drug or treatment, such as a protein, enzyme, or receptor involved in a disease.
- Screening of compounds: Researchers then screen various compounds or molecules to find one that can interact with the target and potentially treat the disease.
- In vitro studies: In vitro studies are conducted in laboratory settings using cells or tissues to test the safety and efficacy of the compound.
- Animal studies: If the in vitro studies are successful, the next step is to conduct animal studies to evaluate the safety and efficacy of the compound in vivo.
- Pharmacokinetics and toxicology: Researchers also conduct pharmacokinetics and toxicology studies to evaluate how the compound is absorbed, distributed, metabolized, and eliminated by the body, as well as to assess any potential toxic effects.
The results of nonclinical research are then used to design human clinical trials, which are conducted in several phases to test the safety and efficacy of the treatment in humans. If the clinical trials are successful, the treatment may then be approved for use by regulatory agencies and marketed to the public.
Altogen Labs Services
Altogen Labs offers a variety of custom laboratory research services that can be adjusted to client needs. Many services can be done with GLP-compliance, but can also be changed to ensure quicker results. Several of the offered services include:
- Over 120 validated CDX and PDX xenograft models – link
- Transfection services, transient and stable – link
- Assay development (ELISA, RT-PCR, Western Blot) and library screening – link
- Pharm/tox and safety IND tests – link
- Generation of stable cell line in 28 days – link
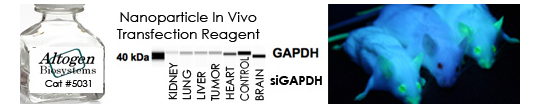
- RNAi experiments (siRNA design, synthesis, validation) – link
- Gene targeting (mRNA knockdown, in vitro and in vivo RNAi validation) – link
- Liposome encapsulation service – link
Altogen Labs provides specialized biotechnology and pharmaceutical services, including generation of stable cell lines, gene silencing and RNA interference services, pre-clinical toxicology IND and other CRO services.