Shop Products
Stable Transfection
Transient and Stable Transfection
The capability to integrate genes into the genome of a mammalian cell has a significant impact on biomedical research. Transfected genetic material can be expressed in the target cells either transiently or permanently depending on the methods utilized and the experimental questions being investigated. Transient transfections are used in most cases to analyze the short-term impacts of an altered gene or induced protein expression. Plasmid DNA (pDNA), messenger RNA (mRNA), short interfering RNA (siRNA), and microRNA (miRNA) are introduced and gene products are expressed in the target cells; however, the nucleic acids do not integrate into the host cell genome, unlike in stable transfections. Gene product expression is transient and typically results in high expression levels that persist for 24-72 hours when RNA is transfected, or 48-96 hours following DNA transfection.
Conversely, in order to analyze the long-term impacts of an altered gene or induced protein expression, investigators typically utilize stable transfection protocols to develop stable cell lines. In a subpopulation of transfected cells, whether the desired effect is a stable or transient transfection, the transfected genetic material will integrate into the genome. In order to create stable cell lines, investigators will take advantage of this natural occurrence, and introduce the gene of interest along with a selectable marker. Growth of transfected cells, in the presence of a selecting agent, will enable the subpopulation of cells where the exogenous genetic material has been incorporated into the genome to persist while the remaining cells undergo selection. Utilizing this method, investigators are able to develop cells that permanently express specific genes through their incorporation in the cellular genome.
Reporter Gene Assays
Reporter gene assays take advantage of the transfection principle and are used to study gene expression, signal transduction, and other cellular activities. “Reporters” (as these genes are commonly referred to) are chosen carefully, depending on their cellular influence and whether they can be efficiently classified and measured. Furthermore, they are regularly used as selectable markers to observe gene expression in genetic studies.
Utilizing reporter genes for research reaps several benefits: they serve as markers, support the accumulation of data from gene expression observation, and provide a valuable resource for reproducibility of experiments. The characteristics of reporter markers frequently include fluorescence and luminescence, both of which can be used for detection purposes.
The most common reporter systems to measure transfection efficiency include using a green fluorescent protein (GFP), Luciferase, and β-galactosidase (β-gal) assays. Cells are transiently or stably transfected with a luciferase expression plasmid using a transfection kit at the appropriate reagent-to-DNA ratio. Luciferase expression is measured 24 hours post transfection using at least 3 replicates for standard deviation control.
Transient and Stable Transfection Protocols
Depending on the final goal of an experiment, different types of transfection may be necessary. Although the technique has been hailed for its versatility in biological applications, there are nonetheless certain barriers that can be encountered in specific circumstances. Generally speaking, transient transfections are far easier to achieve than stable ones, as stable transfections require the alteration of genomic DNA, and not just an introduction of a nucleic acid sample. As a result, stable transfections generally only occur in around 10% of transfected cells, necessitating the selection of viable populations via biological markers or reporter genes.
Other barriers to transfection include the kinds of cells being used for the experiment, along with the circumstances in which it is being conducted. Usually, in vivo transfections are more difficult to achieve than those in vitro. It is rather trivial that transfecting cells within an organism requires a different approach to transfection and an attention to the distribution of transfected compounds. In vitro experiments, though, are generally sufficient for most experimental goals. Protein production through transient transfections only requires cultured cells, while initial drug studies may want to genetically modify cells in vitro for faster results.
Stable Transfection Protocol Tips
- Use high quality reagents and columns to purify your plasmid DNA
- Use high quality reagents and columns to purify your plasmid DNA
- Optimize your pDNA concentration and transfection reagent to DNA ratio
- Do not passage your cells too much
- Transfect fluorescent pDNA positive control (lucifirase, GFP, b-gal)
- Reduce or eliminate antibiotics in the growth media
- Reduce serum concentration in cell growing medium during transfection
- Don’t add selection antibiotic too early, at least wait the 72-96 hours required to allow cells to recover after transfection
Development of stable cell lines by Altogen Biosystems:
There are several different options for engineering stable expression of exogenous genes in cultured cells. The choices made can be made easier by consulting with Altogen Biosystems scientists (please contact us at techserv@altogen.com).
Production of protein or expression of regulatory RNA (i.e. miRNA, shRNA):
- Effects choice of cells to use
- Effects choice of expression vector
- Effects method of screening
Constitutive expression versus inducible expression
- Depends on type of downstream experiments
- Depends on protein properties (i.e. cytotoxicity, anti-mitotic)
- Inducible systems provide superior negative controls
- Inducible systems provide unique downstream experimental options
- Several inducible human cancer cell lines are immediately available
Expression vector: plasmid vs. viral
- Viral vectors require more time for vector development, however, less time for drug selection and screening
- Viral vectors are a good option for hard to transfect cells
- Use of viral transfected “populations” eliminates the need for identifying single cell clones
What cell line(s) to work with
Although the choice of cell line to use is usually not flexible, a key consideration is the efficiency of stable transfection. If a cell line is known to be difficult to transfect or an uncharacterized cell line is being used, then several pilot transfection studies should be performed. A test transfection procedure with multiple transfection methods/reagents can be performed usually within 1 week. Testing includes transfection with a reporter plasmid such as a GFP expression vector and measuring the percentage of transfected cells 48 -72 hours post-transfection. In cases where very low transfection efficiencies (<10%) are observed, viral expression systems should be considered.
Development of stable cell lines (standard service):
Prior to cell transfection:
- The expression vector was designed and constructed according to the client’s specifications. In this case a protein encoding sequence was inserted into the inducible expression vector for protein production
- The target cell line was a readily available inducible cell line
- The screening procedure was determined to be western blot analysis of the inducible expressed protein. Antibodies were identified and validated for this purpose
Stable cell transfection and clonal isolation procedure:
Transfect cells with expression vector. Allow recovery of cells, integration of transfected DNA and expression of drug-selection gene (48 hours).
Dilute cells for drug selection and clonal growth. Limited dilution cloning was used to ensure selection of clonal populations (14-21 days).
Transfected cells were two-fold serially diluted and plated into multiple wells of 96 well microtiter plates. The medium was changed to selective medium, replenished every three days and the wells will be visually inspected daily to assess cytotoxic response. Controls: The same serial dilutions were done with non-transfected cells and subjected to both regular medium and drug containing medium. Drug resistance clones were identified at the time non-transfected controls were observe to have 100% cell death. Only serial dilutions resulted in in less than 100% cell growth per well were picked for expansion. Ensuring cell growth in each well was derived from a single cell.
Transient screening assay: An aliquot of transfected cells was analyzed for expression of the transfected gene. Screening for exogenous gene expression is done using endpoint RT-PCR and gel electrophoresis and the results are reported.
Expansion and cryopreservation of drug resistant clones. Drug resistant clones were expanded and two vials of each clone frozen and aliquots of each clone were used for screening.
Screening for inducible protein expression. Each clone was incubated with doxycycline to induce gene expression. Total protein was extracted and subjected to western blot analysis. Two clones observed to have inducible expression were further characterized (as shown below). Cells were induced with doxycycline for 3, 5 and 7 days. Total protein extracts were made and analyzed by western blotting using antibody that specifically recognized the induced protein. Uninduced cultures were used as the negative control.
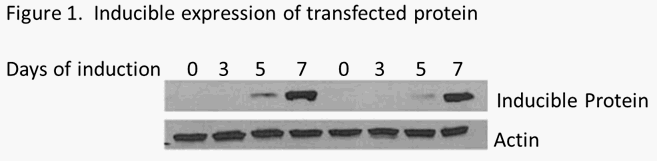
Figure 1. Two drug resistant clones were grown for 7 days under conditions to induce expression of the transfected gene. Cell extracts were made after 0, 3, 5 and 7 days of induction. Western blot analysis was performed to visual the expression of the inducible gene product. The gels were probe with actin antibody to control for any differences in the amount of protein loaded.
Study results and deliverables:
Robust induction of the protein expressed by the transfected expression vector was observed. These two clones were expanded and two aliquots were frozen and delivered on dry ice.
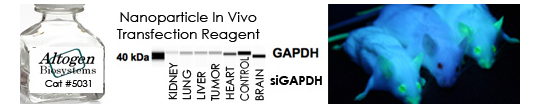
In Vivo Transfection Reagents from Altogen Biosystems
The major challenge in performing RNAi studies in vivo is the effective, directed delivery of functional small RNA molecules into specific tissues. Altogen® In Vivo Transfection Reagents could be conjugated with RNA, DNA, proteins, or small molecules and administered systemically via intravenous (i.v) tail vein injection in order to provide directed gene silencing in specific tissues, including liver, pancreas, kidney, and tumors.
What is Stable Transfection
Stable transfection is a technique used to permanently introduce foreign DNA into a cell line, resulting in stable expression of the introduced gene or genes. This is accomplished by integrating the foreign DNA into the host cell’s genome, typically through the use of viral vectors or plasmids.
Stable transfection involves the following steps:
- Introduction of the foreign DNA: The foreign DNA, typically in the form of a plasmid or viral vector, is introduced into the host cells using a variety of methods, such as electroporation or transfection reagents.
- Selection of stable transfectants: The host cells are treated with a selection agent, such as antibiotics or a toxic chemical, that only allows the growth of cells that have successfully integrated the foreign DNA into their genome.
- Isolation and expansion of stable transfectants: The surviving cells are isolated and expanded to generate a population of stable transfectants that express the introduced gene or genes.
Stable transfection is a powerful tool for studying gene function and regulation, and for the production of recombinant proteins for various applications, such as drug discovery and biotechnology. The stable expression of the introduced gene or genes allows for the study of long-term effects of the gene expression, and can also be used to generate cell lines that are engineered to express a specific gene of interest.
Featured in vivo transfection products from Altogen Biosystems:
In Vivo Lipid Transfection Kit || In Vivo Kidney-targeted Transfection Kit
Altogen Custom Services provide specialized biotechnology and pharmaceutical services, including over 90 validated xenograft models, development of stable cell lines, RNA Interference (RNAi) services, assay development, ELISA and Western Blot services, siRNA library screening and transfection services. Generation of stably-expressing cell lines can be very expensive and time-consuming. Altogen Labs offers generation of stable cell line service completed in just 28 days (see service details).