Description
Purchase Orders: Click “Add to Cart” button to order, then email PO to orders@altogen.com.
Product Availability: In Stock.
LIPID-based In Vivo Transfection Kit for Rodents (Mouse, Rat).
Catalog #5011
- Cationic lipid liposome-based siRNA and plasmid DNA in vivo delivery reagent
- Efficient delivery to the liver, pancreas, kidney, and certain tumor types via systemic administration
- Complete product support information is available here: LIPID In Vivo Transfection Kit
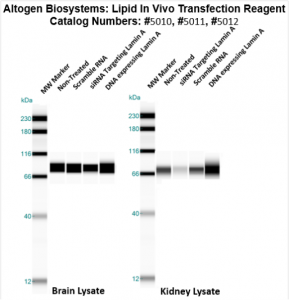
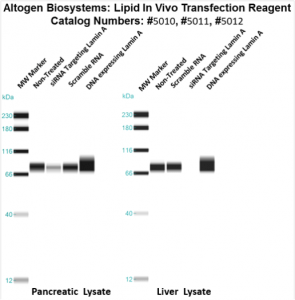
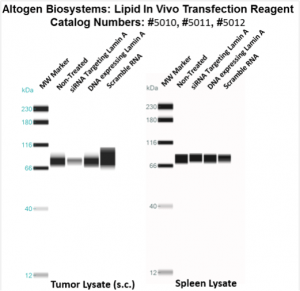
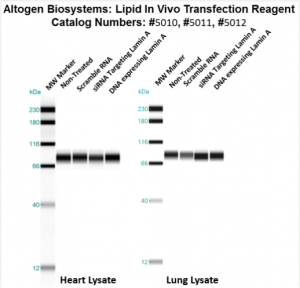
Figure. Systemic administration (i.v.) of Lipid In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA, or non-silencing control siRNA, was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
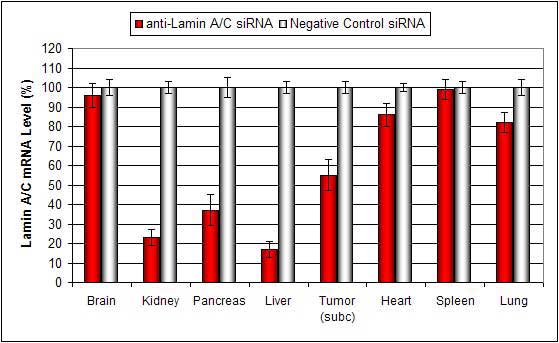
Figure. Systemic administration (i.v.) of a lipid-based in vivo reagent conjugated with siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues were collected, and RNA was isolated 48 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=6).
Selected Lipid Transfection Reagent citations:
- J Biol Chem. 2017 292(2):407-416. Protein Phosphotyrosine Phosphatase 1B in Calpain-dependent … Zhang et al [PDF]
- J Virol. 2015; 89(23):11761-72. Mutational Disruption of cis-Acting Replication Element 2C in Smithee S et al [PDF]
- Virus Res. 2016 Jul 15;220:136-49. Reversion to wildtype of a mutated and nonfunctional coxsackievirus B3CRE(2C). Smithee et al [PDF]
- Nature Biotechnology. 2011 29(4):341-5. Delivery of siRNA to the mouse brain by … Alvarez-Erviti et al [PDF]
-
- Complete data set, ordering, and technical information: Link
POLYMER-based In Vivo Transfection Kit for Rodents (Mouse, Rat).
Catalog # 5021
- Biodegradable polymer-based in vivo reagent
- Efficient delivery to the lung, liver, kidney, and spleen via systemic administration
- Complete product support information is available at: POLYMER In Vivo Transfection Kit
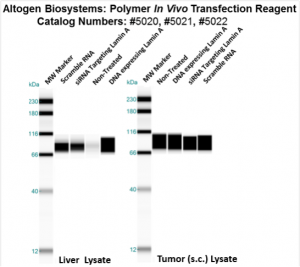
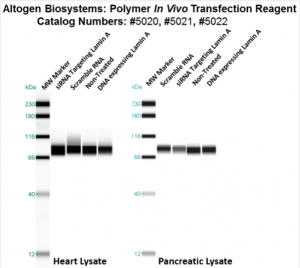
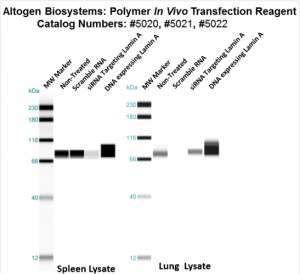
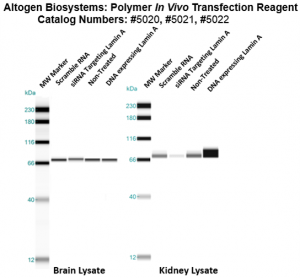
Figure. Systemic administration (i.v.) of Polymer In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
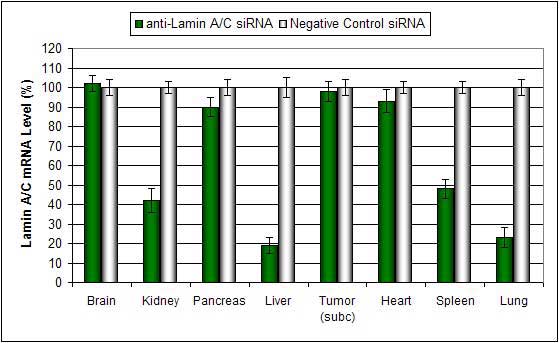
Figure. Systemic administration (i.v.) of a polymer-based in vivo reagent conjugated with siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues were collected, and RNA was isolated 48 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=6).
-
- Complete data set, ordering, and technical information: Link
NANOPARTICLE-based In Vivo Transfection Kit for Rodents (Mouse, Rat).
Catalog #5031
- Nanoparticle-based reagent
- Efficient delivery to the heart, lung, liver, pancreas, kidney, and multiple tumor types via systemic administration
- Complete product support information is available at: NANOPARTICLE In Vivo Transfection Kit
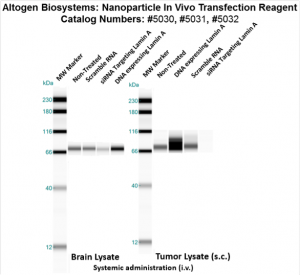
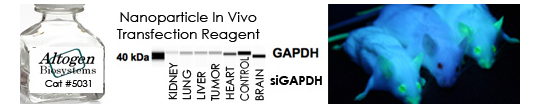
Figure. Systemic administration (i.v.) of Nanoparticle In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA, scrambled sequence non-silencing siRNA control, or a pDNA expression vector encoding Lamin A was performed following the recommended transfection protocol. Nanoparticle-conjugated RNA/DNA complexes were injected at constant pressure into the tail veins of NOD/SCID mice (orthotopic glioblastoma xenograft model developed by Altogen Labs). Seventy-two hours after the first injection, the brain and brain tumor tissues were homogenized and lysed in RIPA buffer supplemented with a protease inhibitor cocktail. A high sensitivity BCA protein assay was used to normalize the protein concentration from each individual sample. Quantitative immunoblotting was performed to analyze changes in Lamin A expression levels using the automated western blot system WES (Protein Simple; San Jose, CA). Images were acquired with the contrast set to white −100 and black 4000 for standardization. Mice treated with scrambled non-silencing siRNA served as controls. Statistical data analysis was conducted using Compass software. Technical replicates (n=10) and biological replicates (n=5) were used. P-value < 0.01.
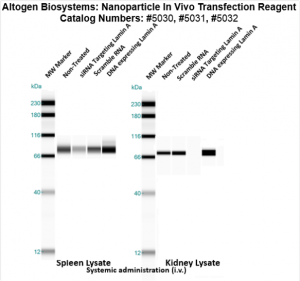
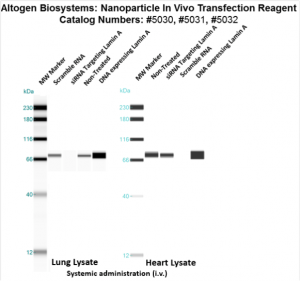
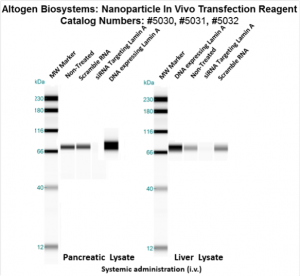
Figure. Intravenous administration of Nanoparticle In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA, scrambled sequence non-silencing siRNA control, or a pDNA expression vector encoding Lamin A was performed following the recommended transfection protocol. Nanoparticle-conjugated RNA/DNA complexes were injected at constant pressure into the tail veins of NOD/SCID mice. Seventy-two hours after the first injection, the spleen and kidney tissues were homogenized and lysed in RIPA buffer supplemented with a protease inhibitor cocktail. A high sensitivity BCA protein assay was used to normalize the protein concentration from each individual sample. Quantitative immunoblotting was performed to analyze changes in Lamin A expression levels using the automated western blot system WES. Images were acquired with the contrast set to white −100 and black 4000 for standardization. Statistical data analysis was conducted using Compass software. Technical replicates (n=10) and biological replicates (n=5) were used. P-value < 0.01.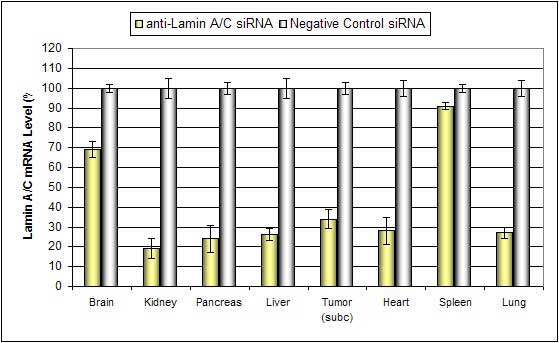
Figure. Systemic administration (i.v.) of a nanoparticle-based in vivo reagent conjugated with siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues were collected, and RNA was isolated 48 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=6).
Selected citations:
- Nature Biotechnology. 2011 29(4):341-5. Delivery of siRNA to the mouse brain by … Alvarez-Erviti et al [PDF]
- Cancer Research. 2011 71(15):5144-53. Inhibition of miR-193a expression by… Iliopoulos et al [PDF]
- RNA. 2010 16(11):2108-19. RNase L releases a small RNA from HCV RNA that refolds … Malathi et al [PDF]
- British Journal of Cancer. 2012 107(3):516-26. TIGAR induces p53-mediated cell-cycle … Madan et al [PDF]
- Hypertension. 2014 63(2):353-61. Tissue transglutaminase contributes to … Liu et al [PDF]
- Mol Cell Biol. 2013 33(7). SCO2 induces p53-mediated apoptosis by Thr845 phosphorylation … Madan et al [PDF]
- Hypertension. 2015 65(2):430-9. Neurokinin 3 receptor and phosphocholine transferase… Parchim et al [PDF]
- PLoS Pathog. 2014 10(10) Exosomes from hepatitis C infected patients transmit HCV … Bukong et al [PDF]
-
- Complete data set, ordering, and technical information: Link
PEG-Liposome In Vivo Transfection Kit for Rodents (Mouse, Rat)
Catalog #5041
- PEGylated liposome based reagent
- Efficient delivery to the spleen, kidney, pancreas, liver, and multiple tumor types via systemic administration
- Complete product support information is available at: PEG-Liposome In Vivo Transfection Kit
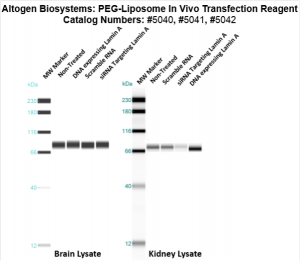
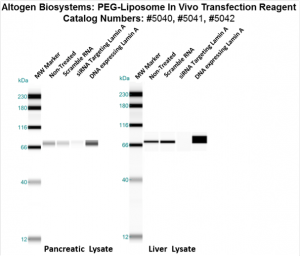
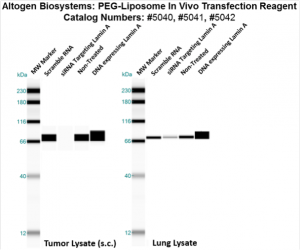
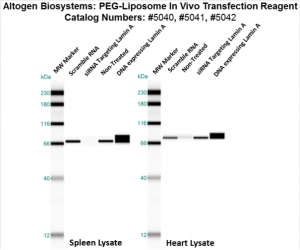
Figure. Systemic administration (i.v.) of PEG-Liposome In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
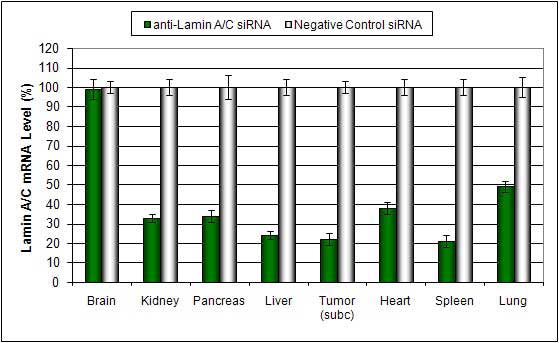
Figure. Systemic administration (i.v.) of a liposome-PEG-based in vivo reagent conjugated with siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues were collected, and RNA was isolated 48 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=6).
- Complete data set, ordering, and technical information: Link
Brain-targeted In Vivo Transfection Reagent
Catalog #5091
- Nanoparticle liposome-based reagent
- IV systemic administration (tail vein)
- Efficient delivery of protein, small molecule, RNA, and pDNA into brain (neurons and glial cells)
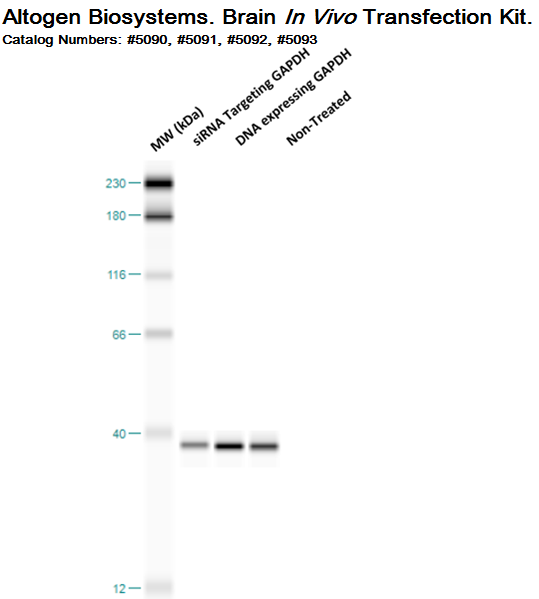
Figure. Systemic administration (i.v.) of Brain In Vivo Transfection Reagent conjugated with (a) 100 µg of chemically modified siRNA targeting GAPDH, (b) pDNA expressing GAPDH, or (c) control was performed following the recommended protocol. Tissues (homogenized brain) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for GAPDH gene expression levels (n=5).
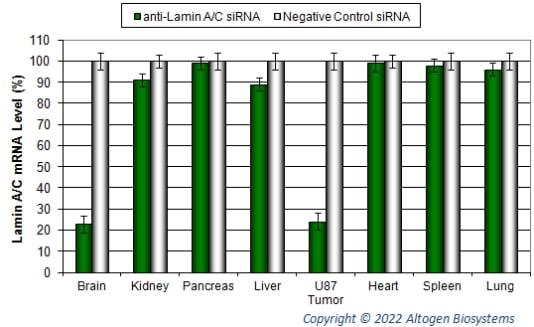
Figure. Systemic administration (i.v.) of Brain In Vivo Transfection Reagent conjugated with 150 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, U87 orthotopic tumor, kidney, pancreas, heart, spleen, and lungs) were collected, and RNA was isolated 24 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=8).
- Complete data set, ordering, and technical information: Link
Lungs-targeted In Vivo Transfection Reagent
Catalog #5081
- Nanoparticle liposome-based reagent
- Intranasal or inhalation administration
- Efficient delivery of biomolecules to the lung tissue and lung tumors
Figure. Aerosol administration of Lung In Vivo Transfection Reagent conjugated with (a) 100 µg of chemically modified siRNA targeting Lamin A/C, (b) no treatment control, or (c) pDNA expressing Lamin A/C was performed following the recommended protocol. Tissues (homogenized lungs) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels (n=7).
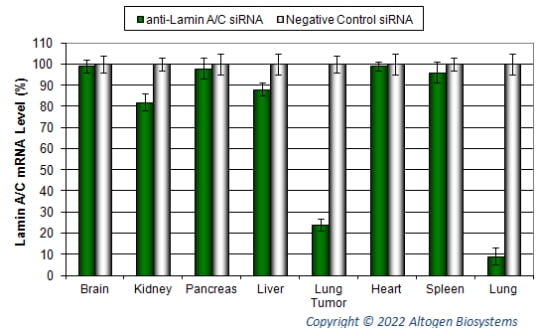
Figure. Intranasal administration of Lungs In Vivo Transfection Reagent conjugated with 2 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol (PDF). Tissues (liver, brain, kidney, tumor, heart, spleen, lung, and pancreas) were collected, and RNA was isolated 24 hours after administration. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=8).
- Complete data set, ordering, and technical information: Link
Pancreas-targeted In Vivo Transfection Reagent
Catalog #5051
- Biodegradable lipid liposome-based reagent
- Efficient delivery to the pancreas tissue (via systemic administration) and pancreas tumors
- Efficient siRNA, shRNA, microRNA, and plasmid DNA delivery
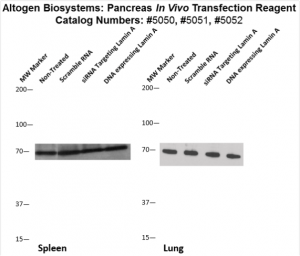
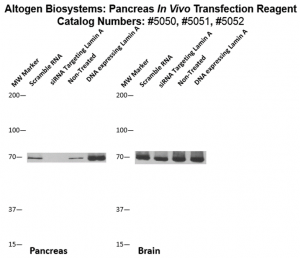
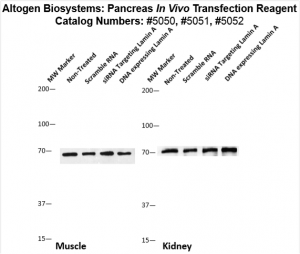
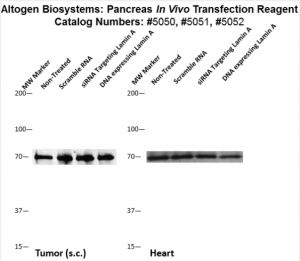
Figure. Systemic administration (i.v.) of Pancreas In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
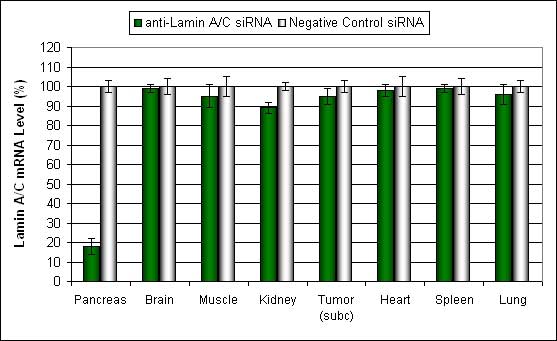
Figure. Systemic administration (i.v.) of Pancreas In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (pancreas, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and RNA was isolated 48 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=8).
- Complete data set, ordering, and technical information: Link
Kidney-targeted In Vivo Transfection Reagent
Catalog #5071
- Nanoparticle-based liposomal reagent
- Efficient delivery to the kidney tissue and kidney tumors
- Functionally validated in mice and rats
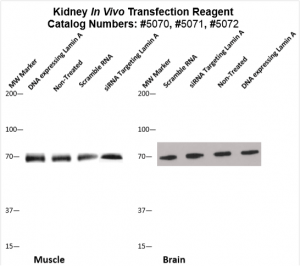
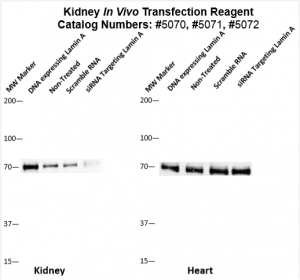
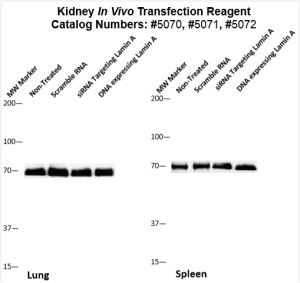
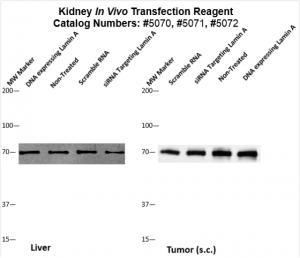
Figure. Systemic administration (i.v.) of Kidney In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
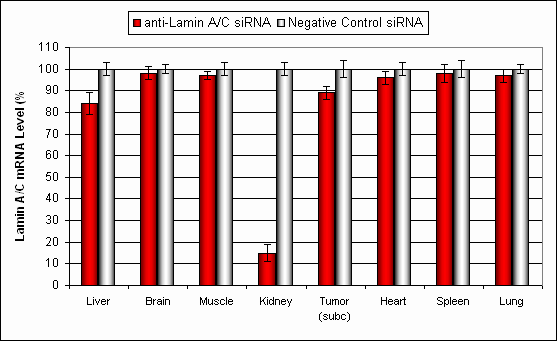
Figure. Systemic administration (i.v.) of Kidney In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and RNA was isolated 24 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=7).
- Complete data set, ordering, and technical information: Link
Liver-targeted In Vivo Transfection Reagent
Catalog #5061
- Biodegradable lipid liposome-based reagent with minimal cytotoxicity
- Efficient delivery to the liver tissue and liver tumors (via systemic administration)
- Efficient siRNA, shRNA, miRNA, and plasmid DNA delivery
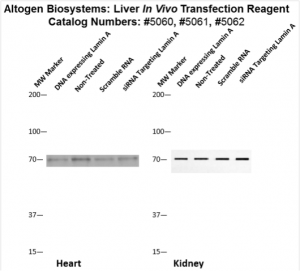
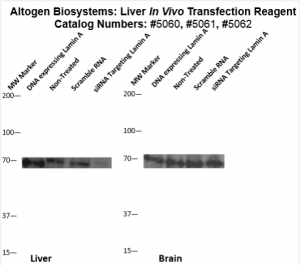
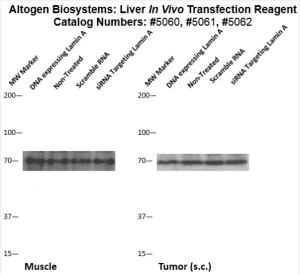
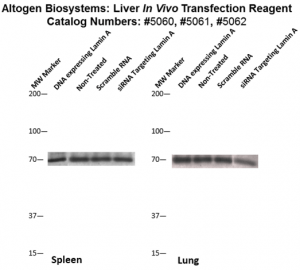
Figure. Systemic administration (i.v.) of Liver In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and the total protein fraction was isolated 72 hours after the first injection. Samples were analyzed by Western blot analysis for Lamin A/C gene expression levels.
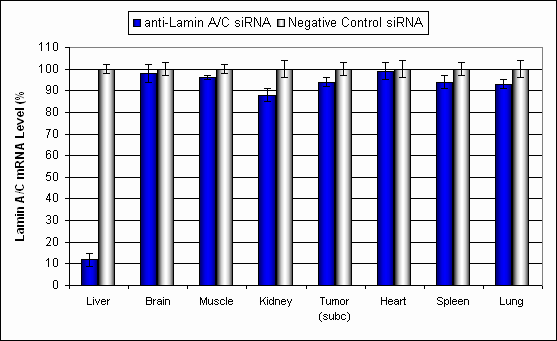
Figure. Systemic administration (i.v.) of Liver In Vivo Transfection Reagent conjugated with 80 µg of chemically modified siRNA targeting Lamin A/C mRNA or non-silencing control siRNA was performed following the recommended protocol. Tissues (liver, brain, muscle, kidney, tumor, heart, spleen, lung) were collected, and RNA was isolated 24 hours after the first injection. Samples were analyzed by qRT-PCR for Lamin A/C gene expression levels. Ribosomal RNA levels were used to normalize the Lamin A/C data. Data are presented as means ± SD (n=6).
- Complete data set, ordering, and technical information: Link
Selected in vivo transfection kits citations:
- Nature. 2008 454(7203):523-7. Innate immunity induced by composition-dependent RIG …Saito et al [PDF]
- RNA. 2010 16(11):2108-19. RNase L releases a small RNA from HCV RNA that refold … Malathi et al [PDF]
- Diabetologia. 2012 55(7):2069-79. The p47phox- and NADPH oxidase organiser … Youn et al [PDF]
- British Journal of Cancer. 2012 107(3):516-26. TIGAR induces p53-mediated cell-cycle … Madan et al [PDF]
- Hypertension. 2014 63(2):353-61. Tissue transglutaminase contributes to … Liu et al [PDF]
- Circulation Research. 2010 15;107(8). Kruppel-like factor-4 transcriptionally regulates … Cowan et al [PDF]
- Hypertension. 2012 59(1):158-66. Role of uncoupled endothelial nitric oxide synthase … Gao et al [PDF]
- Jounal of Biological Chemistry. 2012 287(4):2907. Chaperoning of mutant p53 protein … Gogna et al [PDF]
- PLoS Pathogens. 2012 8(8) Uridine composition of the poly-U/UC tract of HCV RNA … Schnell et al [PDF]
- J Proteome Res. 2012(11) Retinal proteome analysis in a mouse model of oxygen-induced … Kim et al [PDF]
- J Transl Med. 2010 15;8:133. Prevention hyperglycemia-induced myocardial apoptosis … Zhang et al [PDF]
- Mol Cell Biol. 2013 33(7). SCO2 induces p53-mediated apoptosis by Thr845 phosphor … Madan et al [PDF]
- Hypertension. 2015 65(2):430-9. Neurokinin 3 receptor and phosphocholine transferase… Parchim et al [PDF]
- Gastroenterology. 2011 141(2) Differential type I interferon-mediated autophagic … Desai et al [PDF]
- PLoS Pathog. 2014 10(10) Exosomes from hepatitis C infected patients transmit HCV … Bukong et al [PDF]
All Altogen® In Vivo Transfection Kits supplied with ready-to-run transfection protocols that eliminate the need for extensive transfection optimization experiments. Read more about transfection technology at Altogen’s Transfection Resource.
Tissue-targeted In Vivo Transfection Reagents for Rodents (Mouse, Rat):
- LIVER-targeted in vivo reagent
- KIDNEY-targeted in vivo reagent
- PANCREAS-targeted in vivo reagent
- LUNG-targeted in vivo transfection reagent
- BRAIN-targeted in vivo transfection reagent
Altogen Laboratory Research Services:
Altogen Biosystems is a life sciences company that offers cell type-specific and pre-optimized transfection products, elecroporation kits, and in vivo delivery reagents. Advanced formulation of reagents and optimized transfection protocols provide efficient intracellular delivery of protein, DNA, mRNA, shRNA and siRNA molecules. Read more about transfection technology at Altogen’s Transfection Resource. Altogen Labs provides safety and efficacy preclinical research services. GLP-compliant studies for IND applications, and drug development, including over 90 in-house validated xenograft models, safety toxicology, etc (visit AltogenLabs.com).
Purchase Orders: Click “Add to Cart” button to order, then email PO to orders@altogen.com.
Product Availability: In Stock.