Shop Products
Lungs-Targeted Transfection Reagent: Safe and Effective Delivery of Biomolecules
August 2, 2023
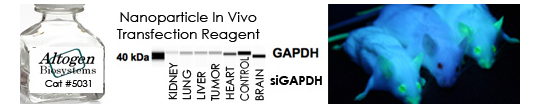
Today we are happy to announce the launch of a new lungs-targeted in vivo transfection reagent. The reagent is crafted to transport small molecules, DNA, RNA, and proteins to the lungs tissue, aiming to treat lung diseases, including cystic fibrosis and lung cancer. The transfection reagent is a non-viral complex comprising nanoparticle-sized cationic liposomes that play a crucial role in facilitating the efficient delivery of cargo biomolecules into lung cells while avoiding the activation of the immune response (transfection protocol and data are now available here).
Lung cancer stands as the primary contributor to cancer-related deaths and ranks among the most frequently diagnosed malignancies worldwide. During the past 14 years Altogen Biosystems successfully engineered in vitro transfection reagents optimized for many lung cancer cell lines (A549, H460, COS7, NCI-H23, 3LL, Calu3, NCI-H1975, 4T1, Calu6, H1975, etc). Building upon this experience, the company has extended its efforts to develop an in vivo lung-targeted delivery agent. In vivo transfection, unlike in vitro transfection which occurs in a petri dish, is the process of delivering cargo molecules into tissue. When it comes to lung-targeted in vivo transfection, there are several challenges due to the organ’s natural defense mechanisms, delivery efficiency, immune response, mucosal clearance, and functionality of cargo biomolecules in vivo.
Altogen Biosystem’s Lungs In Vivo Transfection Kit was functionally validated using seven CDX xenograft models: Huh-7, NCI-H226, A549, Calu-3, H460, NCI-H1975, DMS273 demonstrating that reagent surpasses the inherent deposition mechanism of inhaled aerosol droplets in the lungs, enabling targeting not only on the central airways or lungs periphery but also on specific local regions within the lungs.
About us:
Altogen Biosystems is a leading provider of life science research transfection reagents. Our mission is to provide scientists with the tools needed to make new discoveries and improve human health. Altogen Biosystems’ unwavering dedication to innovation and research, coupled with its broad range of unique products and services, has helped position the company as a leader in the field of in vivo research products.